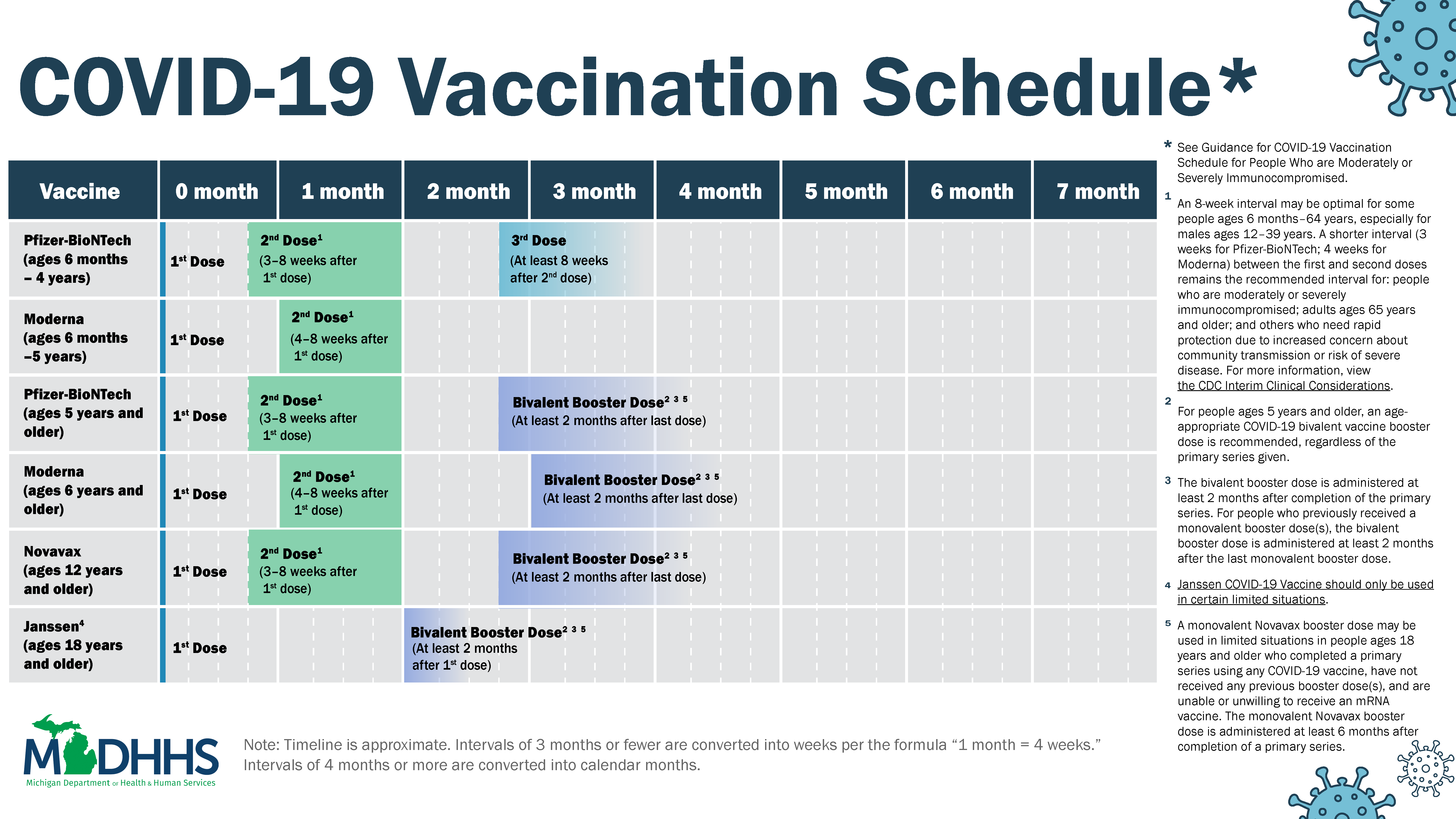
Bivalent vaccine
If bivalent shots are ready for fall infectious disease expert Dr. Walensky urged others to join her in getting the.
New Pfizer Covid 19 Bivalent Vaccine Booster Now Available Through Musc Health Musc Charleston Sc
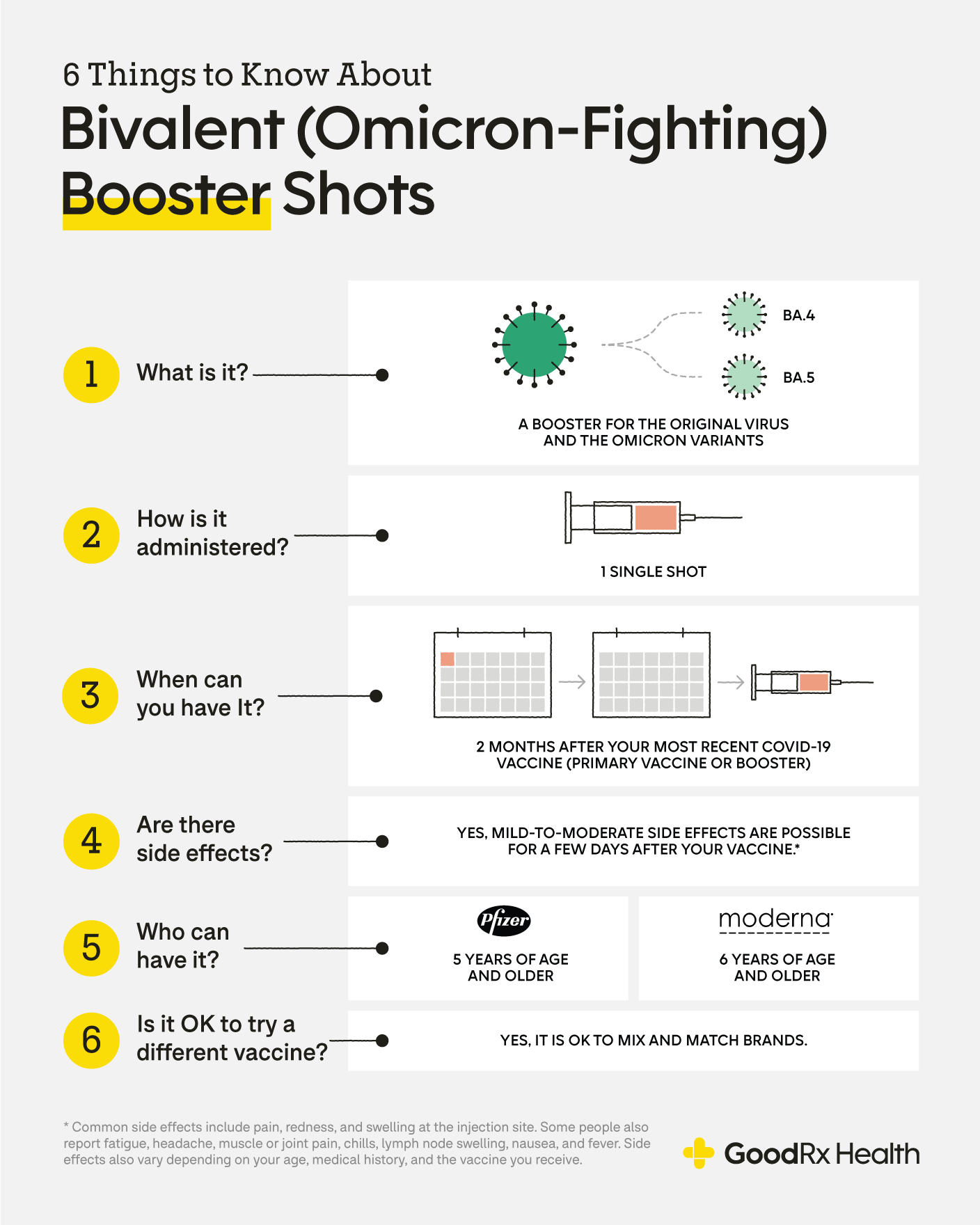
The bivalent booster is the most recent version of the COVID-19 vaccine.
. New bivalent COVID vaccine may not be more protective but its still recommended. Rochelle Walensky became the latest American to get the new bivalent COVID-19 booster shot. A bivalent vaccine elicits an immune response against two different antigens.
The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing. The safety and immunogenicity of the bivalent omicron-containing mRNA-1273214 booster vaccine are not known. The bivalent vaccine which Moderna has said it hopes will be authorized for use in the United States this fall is designed to target both the original omicron variant and the.
Data collected by the FDA for earlier bivalent COVID-19 booster vaccines suggests that these shots successfully provided immunogenicity a boost to your immunity and elicited. A bivalent vaccine however contains mRNA components from two strains of virus. This can means two different viruses or two variations of one virus.
The safety of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine Bivalent for individuals 12 years of age and older is based on safety data from a clinical study. Simply put a bivalent vaccine is a type of vaccine that protects against a combination of two or more coronavirus strains. A booster program a.
Original COVID-19 vaccines which have been authorized and administered to millions of Americans since 2020 are now referred to as monovalent According to the FDA. New York City continues to offer at-home Pfizer vaccinations for New York residents who are homebound or at least 65 years old. Isaac Bogoch expects we could be running three simultaneous vaccine programs.
Pfizers bivalent booster is authorized for those 5 or older while Modernas authorization is for. About 230 million Americans are eligible for a bivalent COVID-19 vaccine booster. Bivalent Covid-19 booster jabs extended to those aged 18 to 49 from Nov 7.
The current COVID-19 vaccines. The updated version of the Pfizer-BioNTech. The FDA authorized bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least two months after completing.
On Thursday CDC Director Dr. The new bivalent COVID-19 vaccine includes mRNA from the original strain of SARS-CoV. Homebound Vaccination Program.
This helps to create a broader immune. Households to get antigen rapid test kits. Kaiser nurse Marilyn Antonio left gives a Moderna booster shot to Ted Naifeh.
It contains both the original vaccine strain of the virus and a strain derived from the BA5. An updated version of the COVID-19 vaccine made by Moderna that targets two coronavirus variants known as a bivalent vaccine has today been. In this ongoing phase 2-3 study we.
Health Canada has approved Pfizers new bivalent COVID-19 vaccine which targets the virus strains now most common in Canada. Based on post market surveillance to date over 11 million updated bivalent mRNA vaccine doses have been administered in the US without any safety concerns reported. If you recall on August 31 the FDA announced that they had amended the original Moderna and Pfizer-BioNTech Covid-19 mRNA vaccine EUAs to include their new bivalent.
They compared virus neutralization by sera collected from individuals vaccinated with three doses of the original monovalent vaccines and a fourth dose of the bivalent vaccine.
Moderna Bivalent Covid Vaccine Appears To Work Against Omicron Subvariants Ba 4 And Ba 5
New Bivalent Covid 19 Booster Shots Are Now Available At Seattle Children S
What S The Most Effective Covid 19 Booster Shot Available Today Goodrx

Omicron Covid 19 Boosters Are Recommended For All Chicago Sun Times
Singapore Grants Interim Approval For Moderna S Bivalent Covid 19 Booster Vaccine Reuters
Moderna Announces Bivalent Vaccine Booster Effective Against Omicron
Moderna Booster Candidate Shows Strong Response Against Omicron Subvariants Reuters

Ccchd Pausing Covid 19 Booster Shots Ahead Of Authorization For Bivalent Vaccine Prevent Promote Protect
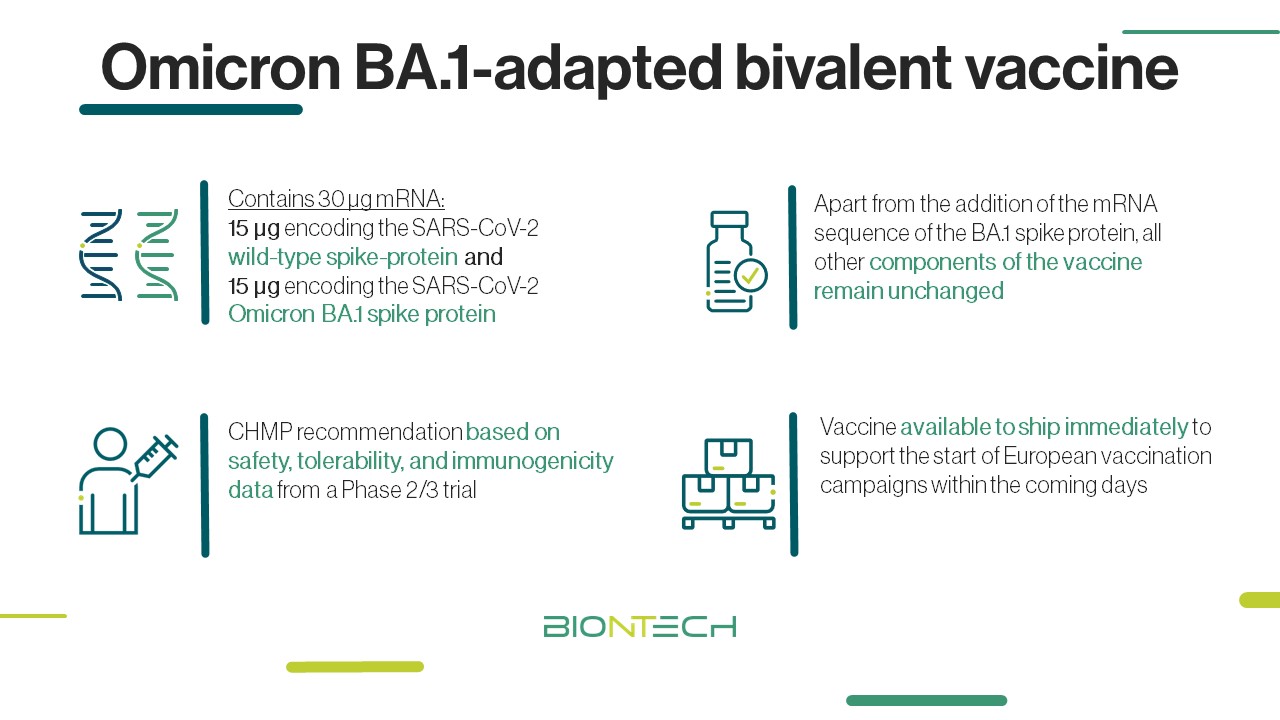
Biontech Se On Twitter Omicron Ba 1 Adapted Bivalent Vaccine Update Our Ba 1 Bivalent Covid 19 Vaccine Was Recommended For Conditional Marketing Authorization By Ema News Committee For Medicinal Products For Human Use As A Booster
New Bivalent Covid Vaccine Booster

Fda Panel Gives Thumbs Up To Omicron Containing Covid Boosters Medpage Today
Pfizer Asks Fda To Authorize Bivalent Covid 19 Vaccine For Children Age 5 11 Aha News

Fda Issues Updated Eua For Bivalent Booster Doses To Fight Omicron Providers To Cease Administration Of Monovalent Boosters Aha News

Bivalent Mrna Vaccine Induces Powerful Antibody Response To Sars Cov 2 Omicron Subvariants

Alarm Over Risk Of Mixing Up Booster And Conventional Vaccine Los Angeles Times
Covid 19 Bivalent Vaccine Available At Uhc Uga Student Affairs
Latest Covid Bivalent Booster Now Available At Qnhch The Queen S Health System